
Phases of Clinical Trials and What Happens in Each Phase
This 4-part series explains what clinical trials are, how they’re run, what all the unfamiliar terminology means, and why people choose to participate in trials. We also include helpful resources to learn more.
Now that you’re familiar with the types of clinical trials and the words used to describe and classify them, let’s dive into the structure of a drug development program. You can think of it as a pathway, with the ultimate destination being your own medicine cabinet.
Every program begins in a lab, where scientists develop and test new ideas. This can include drugs, vaccines, and medical devices. If the results of these tests are promising, the study advances to the next step.
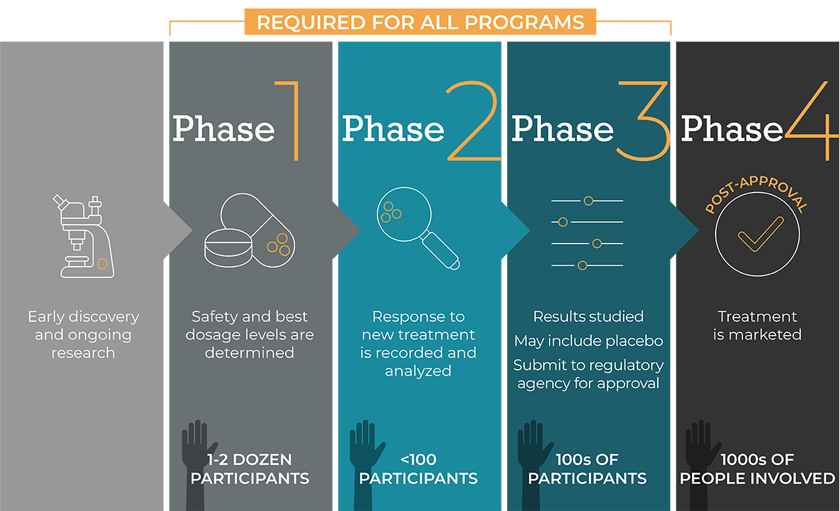
As you can see, a clinical trial is a very stringent process and no step is taken lightly. In the next installment, we’ll talk about what is arguably the most critical part of all of this: the participants. Why would someone agree to enroll in a clinical trial? There is no single answer.
Clinical Trial Resources
If you’re thinking about participating in a clinical trial, PATHFNDR is now enrolling patients to evaluate the safety and efficacy of paltusotine, an investigational drug for the treatment of acromegaly.
CLICK HERE to learn more about the study and the eligibility requirements.

- This database of privately and publicly funded clinical studies conducted around the world is one of the best resources available. You can search by disease, country, and other key filters to find a trial that may be right for you. The site includes a good list of questions to ask when considering participating in a clinical trial. See them here.
Deciding to participate in clinical trials
- Animation and easy-to-understand language make this video from the Office for Human Research Protections (OHRP) a must-see for any potential trial participant.
- Another OHRP video addresses randomization. Watch it here.
A participant’s first-hand account
- Watch Juliana’s story about her experience participating in a trial for sickle cell anemia
References
National Institutes of Health (2015, August 20) Clinical Trials [https://www.nih.gov/research-training/clinical-trials]. Retrieved May 2020.
U.S. Food and Drug Administration (2018, January 04) Step 3: Clinical Research [https://www.fda.gov/patients/drug-development-process/step-3-clinical-research]. Retrieved May 2020.
ClinicalTrials.gov (2019, March) Learn About Clinical Studies [https://clinicaltrials.gov/ct2/about-studies/learn]. Retrieved May 2020.
National Health Service (2019, May 08) Clinical trials [https://www.nhs.uk/conditions/clinical-trials/]. Retrieved May 2020.
UptoDate. Patient education: What are clinical trials? (The Basics). Topic 16168 Version 11.0



